Examples of chemical properties include flammability toxicity acidity reactivity many types and heat of combustion. The more properties we can identify for a substance the better we know the nature of that substance.
 Chemical Properties Alkali Metals Reactivity With Water Demo Youtube
Chemical Properties Alkali Metals Reactivity With Water Demo Youtube
Iron for example combines with oxygen in the presence of water to form rust.

Is reactivity with water a chemical property. Hydrolysis and oxidation are two such reactions and are both chemical changes. Heat of combustion reactivity with water PH and electromotive force. The change of one type of matter into another type or the inability to change is a chemical property.
Thus reactivity with oxygen is a chemical property. Some water-reactive substances are also pyrophoric like organometallics and sulfuric acid and should be kept away from moisture. This means that wherever water goes either through the ground or through our bodies it takes along valuable chemicals minerals and nutrients.
Pure water has a neutral pH of 7 which is neither acidic nor basic. Examples of chemical properties are. The more properties we can identify for a substance the better we know the nature of that substance.
Examples of chemical properties include flammability toxicity acidity reactivity many types and heat of combustion. Some of the solute dissolves within the mixture but the rest sinks to the bottom the water. There is no simple way to extract the gas back out of water.
An oxidation-reduction otherwise known as redox is a type of reaction that involves the transfer of electrons between two reactants. Indeed water as found in nature almost. These reactions were i.
Chromium does not oxidize Figure 2. Waters Physical Properties Also See. These properties can then help us model the substance and thus understand how this substance will behave under various conditions.
This allows it to be the solvent of life. Dry HCl gas is neutral but its solution in water is a powerful acid. When adding to the solvent water the Nacl spreads throughout.
Water is called the universal solvent because it dissolves more substances than any other liquid. Heat of combustion reactivity with water PH and electromotive force. Chemical stability refers to whether a compound will react with water or air chemically stable substances will not react.
This indicates how strong in your memory this concept is. In each of these reactions the reactant undergoes a change in its chemical composition and new substances are formed. Other physical properties such as the melting temperature of iron or the freezing temperature of water can only be observed as matter undergoes a physical change.
Examples of chemical properties are. Is reacting with water a chemical property. Describes characteristics of matter that may or may not allow them to undergo chemical changes.
For example HCl is very soluble in water but also reacts with water in such a way that it protonates water. Chemical Properties and Chemical Reactions. Water-reactive substances are those that spontaneously undergo a chemical reaction with water as they are highly reducing in nature.
You have to test a substance by making it undergo a reaction to find out these properties. That reaction or lack thereof in the case of water is a very basic way of telling us about the important chemical property of toxicity. Water H2 O is a polar inorganic compound that is at room temperature a tasteless and odorless liquid nearly colorless with a hint of blueThis simplest hydrogen chalcogenide is by far the most studied chemical compound and is described as the universal solvent for its ability to dissolve many substances.
Notable examples include alkali metals sodium through caesium and alkaline earth metals magnesium through barium. And hence by the definition of chemical and physical properties radioactivity is a chemical property. This solubility involved a chemical change.
Other chemical properties include a substances pH value and reactivity with water and oxygen.
All reactions are to some extent reversible. Substances are either chemical elements or compounds.
 Examples Of Chemical Reactions In Everyday Life
Examples Of Chemical Reactions In Everyday Life
A substance formed by a chemical reaction bond energy amount of energy needed to break a bond between two particular atoms.
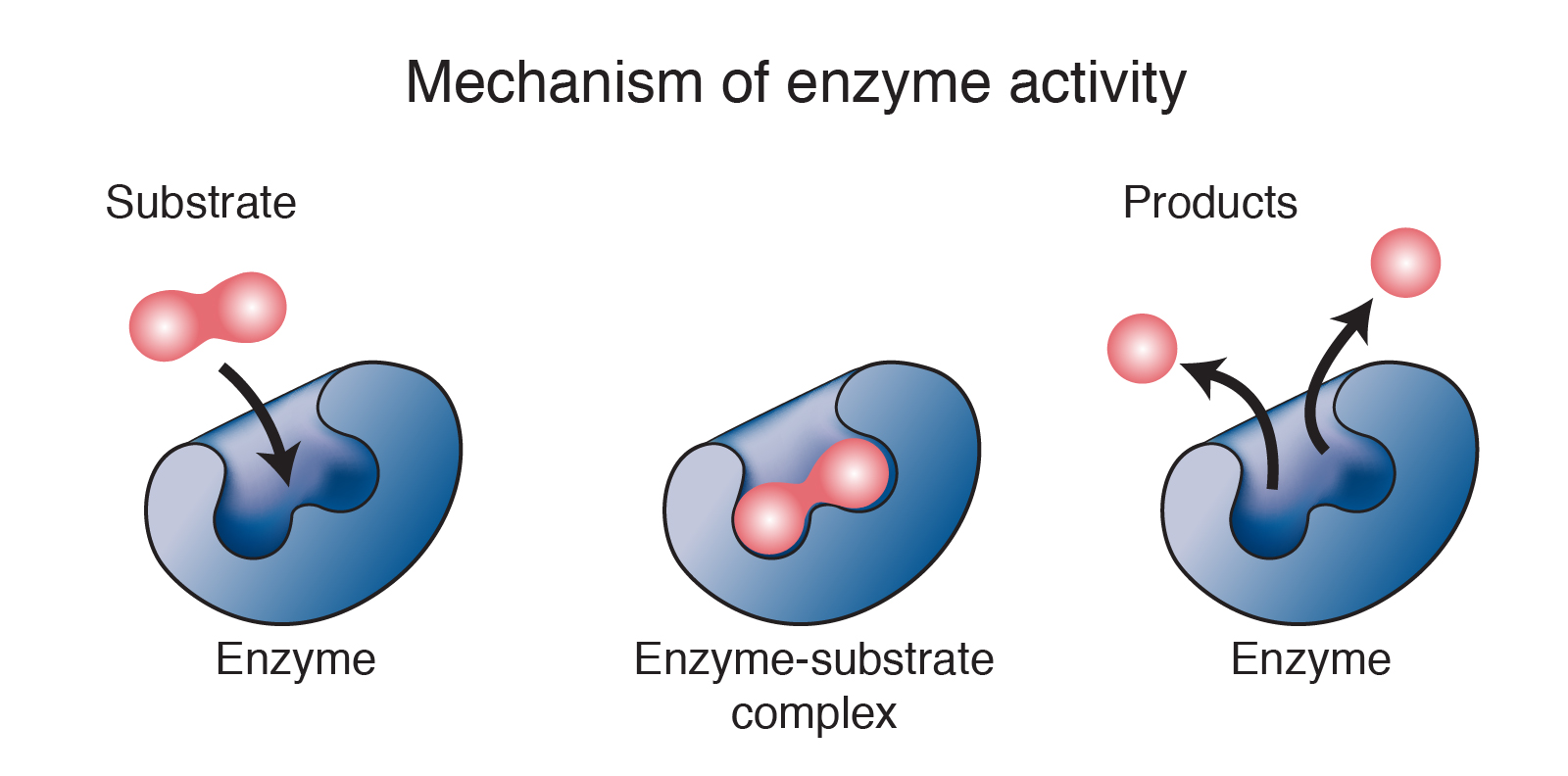
Chemical reaction definition biology. A chemical reaction is a process in which one or more substances also called reactants are converted to one or more different substances known as products. Chemical reactions occur when chemical bonds between atoms are formed or broken. In most biological reactions enzymes act as catalysts to increase the rate of a reaction.
These are the substances that. The products can also react to give the original reactants. By contrast reactions where chemical bonds are formed are often endergonic.
The substances that go into a chemical reaction are called the reactants and the substances produced at the end of the reaction are known as the products. That is the final reaction. Reactions where chemical bonds are broken releasing the energy in those bonds are often exergonic reactions.
First what we start with are the reactants. A chemical reaction is a chemical change which forms new substances. Biological systems are based upon elementary mono- and bimolecular chemical reactions.
ATP stores energy in the form of phosphate bonds. Overall chemical reactions occur only in one direction. Cells must obey the laws of chemistry and thermodynamics.
A common example of a coupled reaction is the formation of ATP a nucleotide that contains chemical energy that is broken down for metabolic uses. A chemical reaction rearranges the constituent atoms of the reactants to create. The field of biochemistry demonstrates that knowledge of chemistry as well as biology is needed to understand fully the life processes of organisms at the level of the cell.
These reactions where chemicals are broken down are called catabolism the destructive part of metabolism. Cell - Cell - Coupled chemical reactions. In order to definitely clarify all necessary conditions for bistability we here present the corresponding minimal system.
In summary a chemical reaction is a process that converts one or more substances to another substance. Chemical reaction definition is - a chemical change that occurs when two or more substances combine to form a new substance. To better understand chemical reactions it will help if we first work through the various parts of the reaction itself.
When two molecules react with each other inside a cell their atoms are rearranged forming different molecules as reaction products and releasing or consuming energy in the process. Biochemical reactions are chemical reactions that take place inside the cells of living things. A chemical reaction occurs when reactants are joined together to create a product that has different chemical properties than the original reactants.
A chemical reaction may be represented by a chemical equation which indicates the number and type of each atom as well as their organization into molecules or ions. Or the amount of energy released when a bond forms. However in many cases the extent of this back reaction is negligibly small and the reaction is regarded as irreversible.
The sum of all the biochemical reactions in an organism is called metabolism. Chemical reactions start with reactants and convert them into products. A coupled reaction is a reaction with a common intermediate that results in energy being transferred from one side of the reaction to the other.
A change in which one or more chemical elements or compounds the reactants form new compounds the products.