Models of the atom M Fowler. Timeline Of The Evolution Of The Atomic Theory BCE Democritus and Leucippus Create First Atomic Theory 460-370 BCE 460 BCE.
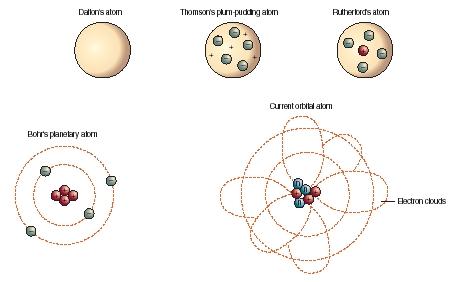 Atomic Model Development Uachemistry13
Atomic Model Development Uachemistry13
History of Atomic Theory Cathode Ray Tube Electricity is passed through a glass tube filled with gas.

The development of atomic theory. The atom consists of three main particles 1. Here are the postulates of Daltons atomic theory. Start studying Development of the Atomic Theory.
This is called the nucleus of the atom. Most of the matter of the atom is found in a very small part of the atom. His book A New System of Chemical Philosophy Part I 1808.
These atoms couldnt be destroyed or created only rearranged and combined in different ways. The Proton which has mass of approximately 1 atomic mass unit and a positive charge. Daltons atomic theory also stated that all compounds were composed of combinations of these atoms in defined ratios.
The Greek philosophers Democritus and Leucippus theorized that the world was made up of tiny particles called atoms. Daltons atomic theory proposed that all matter was composed of atoms indivisible and indestructible building blocks. Atomic Structure Periodicity and Matter.
Rutherfords Revised Atomic Theory 1911 Result. The gas beam can be bent with a magnet. 3580 for a 2-page paper.
While all atoms of an element were identical different elements had atoms of differing size and mass. Learn vocabulary terms and more with flashcards games and other study tools. He developed the atomic theory because he disagreed with the theory of atoms that Aristotle had previously proposed.
Development of the Atomic Theory Early Atomic Theory Although the idea of the atom was first suggested by Democritus in the fourth century BC his suppositions were not useful in explaining chemical phenomena because there was no experimental evidence to support them. Hire a subject expert to help you with Development of Atomic Theory. In the early 19th Century John Dalton proposed his atomic theory.
Development of Atomic Theory The atomic theory which holds that matter is composed of tiny indivisible particles in constant motion was proposed in the 5th cent. BC by the Greek philosophers Leucippus and Democritus and was adopted by the Roman Lucretius. He passed through several experiments and discovered several atomic.
It is very tiny and extremely dense. Some of the positively charged particles were deflected or even. The Neutron which has a mass of approximately 1 atomic mass unit and no charge.
Matter is composed of exceedingly small particles called atoms. First published in 1807 many of Daltons hypotheses about the microscopic features of matter are still valid in modern atomic theory. John Dalton developed the atomic theory around the 1800s.
Most of the positively charged particles went straight through the gold foil. This matter may be. The basic idea behind all element theories is that the matter that surrounds us is composed of more simple matter.
English chemist and physicist John Dalton extended Prousts work and converted the atomic philosophy of the Greeks into a scientific theory between 1803 and 1808. Part II 1810 was the first application of atomic theory to chemistry. An atom is the smallest unit of an element that can participate in a chemical change.
Development of Atomic Theory Since the ancient times humans thought of explaining the material world in all of its complexity. However Aristotle did not accept the theory and it was ignored for many centuries. Atoms consist of charged particles The negatively charged particles are called electrons 1897 The positively charged particles are called protons 1920 Plum Pudding or Chocolate Chip Cookies.
Matter came in a variety of elements and all the atoms of a given element were identical in mass and their other properties. Development of atomic theory The concept of the atom that Western scientists accepted in broad outline from the 1600s until about 1900 originated with Greek philosophers in the 5th century bce. History and philosophy of science through models.
The development of the atomic model R Allain Wired.