Latent Heat of Fusion of Ice Cream 21014 Jkg 2. LatentHeatofFusionofIce 237 mentsontheheatoffusionoficerangefrom7941to7991with ameanvalueof7961calories.
WhereQ is heatm is mass andLf is the latent heat of fusion.
Latent heat of fusion of ice. Fusion and Evaporation Heat of common Materials - Melting points heat of fusions boiling points and heat to evaporate common substances - like hydrogen water gold and more. Calculate the latent heat of fusion L for each of the three trials you performed. 1a the effective latent heat of fusion of ice can be calculated as 1 L e L ice Δ H Δ Q x ini 1 P i n i 100 P ini L ice Δ H Δ Q x fin P ini Here x ini is the initial concentration of aqueous solution and P ini is the initial IPF which is the mass fraction of ice in the icesolution mixture.
Q 25 gx 334 Jg q 8350 J. Q mΔH f. Assume that the energy lost by the warm water is gained by the ice but notice that the energy is used first to melt the ice then to raise the temperature of the melted ice to match the water the final temperature of water melt ice is the same.
Q 25 gx 80 calg q 2000 cal. I of the ice. Initial joulemeter reading E 2 299425 J Final joulemeter reading E 1 359205 J Energy supplied by the heater DE E 2 - E 1 5978 J The specific latent heat of fusion of ice is.
Experiment Results Mass of empty beaker 968 g Mass of beaker water 1149 g Mass of water collected m 1149 g - 968 g 00181 kg. Heat of fusion should be a. The heat of fusion or vaporization can be expressed mathematically as Q mL 2 where L is the latent heat of fusion or vaporization depending on the phase transition that occurs.
The known value for the latent heat of fusion of ice is 80 caloriesgram so the measured value below compares pretty well. In this experiment the water and the inner aluminum Calorimeter cup lose energy and cool down while the ice gains energy first in melting and then in warming that melted ice-water up to the final equilibrium temperature of the system. The amount of heat required to melt 25 grams of ice is 8350 Joules or 2000 calories.
If this occurs in an insulated container then the heat gained by the ice is equal to the heat. Heat lost by water equals the mass times the specific latent heat fusion and the equation is VtI ML M is mass of water in difference t stands for the time it takes to heat the ice and L is the latent heat fusion of ice. 2 J 1 0 3 k g 1 3 3 6 1 0 3 J k g 1.
For ice the latent heat of fusion is 80 Cal g -1 in CGS-system. By knowing the masses of the ice the water and the calorimeter and the resulting temperature change after the ice melts the latent heat of fusion of ice is found. Thermal Conductivity of Ice Cream at -26C 993 Wm-K.
Measurement of the specific latent heat of fusion of ice. Its just as easy to express the heat in terms of calories. The calculation of the specific latent heat fusion of ice is as follows.
Specific Heat of Ice Cream at -26C 1629 Jkg-K 5. Thermal Conductivity of Ice Cream at 4C 694 Wm-K 6. Latent heat of fusion of ice 8 0 c a l g 1 8 0 4.
Density 568 kgm3 3. The final steady temperature of the water and ice mixture is found to be 20C. Take c to be 42 Jg-1C-1.
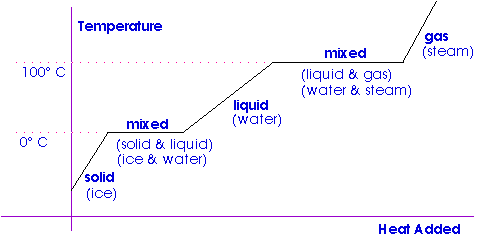
Determination of Latent Heat of Fusion of Ice by the Method of Mixture Let us consider a dry and clean calorimeter with a stirrer of mass m 1. THEORY When heat is added to a substance a temperature change is generally observed to occur. A small amount of ice is placed in a calorimeter containing water.
Specific Heat of Ice Cream at 4C 2948 Jkg-K 4. After giving a brief review of previous determinations of the latent heat of fusion of ice the authors describe in detail their investigation of this constant by two independent calorimetric methods. The first of these is the ordinary method of mixtures.
When ice at zero degrees celsius is added to warm water it first melts and then its temperature increases as it takes heat from the water. Ice - Thermal Properties - Thermal and thermodynamic properties of ice - density thermal conductivity and specific heat at temperatures from 0 to -100 o C. In this experiment an ice cube of mass mt assumed to be at 0oC is placed in a calorimeter containing a mass of.
When all ice melts in an icesolution mixture as shown in Fig. In an experiment to measure the specific latent heat of fusion of ice 20g of crushed ice is added to a beaker of water of mass 160g not including mass of beaker at temperature 30C.